You may have thought you were done with organic chemistry after your college undergraduate classes but—surprise!—you will see it on the MCAT.
The three big questions about organic chemistry on the MCAT are:
- Where does organic chemistry show up on the MCAT?
- How much organic chemistry is on the MCAT?
- What specific organic chemistry topics are tested on the MCAT?
1. Where Does Organic Chemistry Appear on the MCAT?
Organic chemistry content will appear in two sections on the MCAT.
- First, it will appear in the Chemical and Physical Foundations of Biological Systems section , where about 15% of the 59 questions relate to organic chemistry.
- Second, it will appear in the Biological and Biochemical Foundations of Living Systems section , where about 5% of the 59 questions relate to organic chemistry.
Only about 12 of the 230 MCAT questions are directly based on organic chemistry. However, a good foundation in organic chemistry principles will not only help you with those questions but with navigating complex biological structures and deciphering biochemical processes across the entire test.
Note : You will rarely be required to go into the level of detail that you did in your undergraduate organic chemistry classes.
2. How is Organic Chemistry Typically Represented on the MCAT?
First, let’s talk about how you will be asked to apply your organic chemistry knowledge on the MCAT.
-
You will NOT need to perfectly memorize and regurgitate reaction mechanisms or conjure up intricate reaction products after following an endless reaction scheme.
-
You WILL need to rely on a solid foundation in the fundamentals along with an ability to quickly relate that knowledge to information presented inside a passage or a question.
The MCAT is known for layering subjects so that you have to apply reasoning across closely related concepts. In this case, you will need to demonstrate an understanding of organic chemistry concepts and principles in relation to biochemical processes and structures (as seen in the sample questions at the end of this article). The MCAT is testing your ability to locate important information within a passage, reaction, or structure.
Biological structures are a vast subject area, but don't let that intimidate or distract you. Instead, use practice problems to practice focusing your attention and quickly reading paragraphs to extract the important details. This will also improve your content background.
3. What Organic Chemistry Do You Need to Know for the MCAT?
If you’ve taken a two-semester course in organic chemistry, then you’ve likely seen more than what the MCAT will test. It’s important to know what you’re responsible for studying.
Here are the five broad categories of organic chemistry content you will need to focus on:
Stability of Organic Molecules
The concept of stability is a major one in organic chemistry, and it relates to many other concepts that will be tested. The golden rule you must know is that stability is inversely related to reactivity. This means that the more stable a substance is, the less reactive it will be.
Your ability to predict the stability of a molecule will be based on identifying the presence of stabilizing principles like:
- Induction
- resonance
as well as destabilizing influences like:
- ring strain
- steric strain
- etc.
For example, a charge within a molecule often epitomizes instability, but if that molecule possesses the ability to mitigate that charge, perhaps via resonance, it may become sufficiently stable. This is the foundation for predicting important reactivities in organic compounds like:
- acidity
- nucleophilicity
- leaving group tendency
These details will relate more directly to your ability to learn and identify organic reactions that may appear on the MCAT.
Structure
Being familiar with functional groups in organic molecules is an integral prerequisite to being able to examine a structure and identify its most important features.
The MCAT prioritizes the following compounds above others, as these are most likely to appear within biological molecules:
- Alcohols
- Aldehydes
- Ketones
- Carboxylic acids
- Acid derivatives
- Polycyclic and heterocyclic aromatic compounds
You will also need to be familiar with nomenclature for those functional groups and their properties, which will pay off when learning the reactions they participate in.
Isomers are the various molecular structures that may be created from a single molecular formula. It is important to learn the various subdivisions found within this umbrella term, which is where details emerge.
Test-takers must be familiar with three major isomer types:
- Structural isomers:
- Enantiomers
- Diastereomers
- Cis-trans isomers
- Meso compounds
- Conformational isomers
- Stereoisomers
It is also important to be comfortable doing the following:
- Identifying chiral centers in a molecule
- determining absolute configurations (R/S)
Lab Techniques
There are two classes of laboratory techniques that may be tested concerning organic molecules:
- Separation techniques allow the separation of a compound of interest from within a mixture of compounds. These can largely be classified as chromatography techniques, of which there are several types:
- thin-layer chromatography
- high-performance liquid chromatography
- gas chromatography
- size-exclusion chromatography
- ion-exchange chromatograph
- affinity chromatography
- electrophoresis
- liquid-liquid extraction
- distillation
- resolution of enantiomers
For each technique, it is crucial to know the basis for separation (e.g., polarity, charge, size) and the order separation is expected to occur in.
- Diagnostic techniques elucidate the structural features of a compound. They will test your familiarity with a small range of absorption spectroscopy techniques, including:
- IR spectroscopy
- 1 H-NMR spectroscopy
- UV-Vis spectroscopy
- Mass spectrometry
For each technique, it is imperative that you know what information each form of spectroscopy provides about a molecular structure and that you know common characteristic absorptions.
Note: In most cases, undergraduate organic chemistry classes go much deeper than the MCAT will when testing content from this area.
Organic Reactions
Organic Reactions can be split into three major categories on the MCAT:
- Nucleophilic substitution reactions are most likely recalled via the terms S N 1 and S N 2.
- Nucleophilic addition reactions are those that occur primarily at aldehyde and ketone functional groups, which includes:
- aldol condensation
- acetal formation
- Nucleophilic addition-elimination reactions are those that occur at carboxylic acid and acid derivative functional groups.
Many reactions touch on carbonyl chemistry, which is one of the most important functional groups in biology and therefore likely to be tested.
Other important organic reactions include redox reactions and acid-base reactions , largely as they pertain to alcohols and carbonyl-containing compounds.
Structure & Reactivity of Biological Compounds
MCAT test-takers consistently say the same thing: know your amino acids ! Amino acids are aggressively tested on the MCAT, especially in the context of biochemistry, so it’s important to be very familiar with their details.
For all 20 canonical amino acids, you will need to know:
- side chain structures
- classification (polar, nonpolar, acidic, basic),
- 1- and 3-letter abbreviations
- p K a values (where relevant)
You must also be able to apply these details within the context of peptides and proteins. For example, you may be asked to determine the overall charge on a peptide in solution, or to predict the consequence of an amino acid site-mutation in the function of a protein. Scroll down for examples of some amino acid-based practice problems.
From the standpoint of structure and reactivity, familiarity with the following is necessary:
- carbohydrates
- lipids
- nucleic acids
Conquering MCAT Organic Chemistry
The key to success in organic chemistry content on the MCAT is to build a solid foundation in the fundamentals. Exposure to organic chemistry practice problems is a necessary part of this foundation that takes time and can’t be rushed. Over time, you will become more comfortable examining molecular structures for their vital parts, which is routine for organic structures presented on the MCAT.
One last thing to keep in mind: as your grasp of organic chemistry concepts grow stronger, so will your grasp of biochemistry concepts and processes, so there is a significant payoff for the time you invest in prepping organic chemistry for the MCAT beyond what you might expect.
MCAT Organic Chemistry Example Questions (solutions available after the questions):
- Digoxin, a natural product isolated from a variety of foxglove plant, is used to treat atrial fibrillation and other heart conditions.
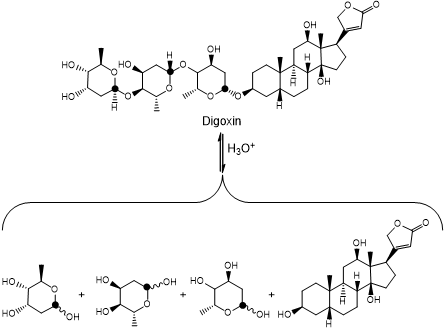
How many fragments are expected to result via acid-catalyzed hydrolysis of digoxin?
- 3
- 4
- 5
- 6
- A mixture containing five amino acids Asp, Phe, Met, Trp, and Tyr was separated using reverse-phase HPLC. What is the expected order of increasing retention time of the components of this mixture?
- Met < Tyr < Asp < Phe < Trp
- Trp < Phe < Asp < Met < Tyr
- Asp < Tyr < Met < Phe < Trp
- Trp < Phe < Met < Tyr < Asp
- Echothiophate is an ocular antihypertensive drug that acts as an irreversible inhibitor of acetylcholinesterase, an enzyme that relies on a nucleophilic serine residue to hydrolyze the neurotransmitter acetylcholine. Which of the following serine substitutions in acetylcholinesterase would most improve the kinetics of reaction with echothiophate?
- K
- D
- M
- C
Solutions to MCAT Organic Chemistry Sample Questions:
- B The key to quickly solving this question is to recognize that digoxin contains a carbohydrate basis for its structure, where only acetal sites (a carbon bonded to two OR groups) will be cleaved in an acid-catalyzed hydrolysis. Digoxin contains three acetal carbons [indicated by dark circles
(“ ”) in the drawing shown below], which reveals that four fragments will result upon hydrolysis. This process is summarized below. Note that it is unnecessary to draw or identify any of the fragment structures shown when solving this question.
- C Solving this question efficiently relies on recognizing the ranking-style associated with the answer choices, knowledge of amino acid classification, as well as the principles of reverse-phase HPLC. Since reverse-phase HPLC utilizes a nonpolar stationary phase and moderately polar aqueous solvent (mobile phase), amino acids with nonpolar (hydrophobic) side chains are expected to strongly adhere to the stationary phase and thus experience the longest retention times (i.e., these species are eluted last). Thus, Asp and Tyr are expected to display the shortest retention times under reverse-phase HPLC conditions and are among the first to be eluted from the column (eliminate choices B and D which show Asp and Tyr eluting last). Met, Phe and Trp, all contain nonpolar side chains and are expected to be the last of the five amino acids to be eluted. Choice A indicates incorrectly that Met (less polar) is expected to be eluted more rapidly than either Tyr or Asp (more polar) and should be eliminated, leaving choice C as the correct answer. Note that knowledge of the exact elution order is unnecessary to solve this question.
- D The question indicates that a reaction between echothiophate and acetylcholinesterase involves a nucleophilic serine residue. If this serine residue is altered to a more nucleophilic residue, the reaction will occur faster. The best approach to solving this question lies in process of elimination. Changing serine to lysine (K) would slow the reaction; lysine is not nucleophilic due to the nitrogen atom lacking a lone pair at physiological pH (choice A is incorrect). A substitution to aspartate (D) will slow the reaction because the negative charge on aspartate is delocalized through resonance, decreasing nucleophilicity (choice B is incorrect). A substitution to methionine (M) would not be as effective as a substitution to cysteine, because unlike methionine, cysteine can be deprotonated to form a highly nucleophilic thiolate anion (choice C is incorrect). Lastly, changing serine to a cysteine (C) will increase the rate of the reaction because sulfur is a more nucleophilic atom than oxygen due to increased polarizability (choice D is correct). Note that although the protonated form of cysteine is favored over the deprotonated form, there will always be some thiolate present at equilibrium, which makes cysteine overall more nucleophilic than methionine. Furthermore, because methionine has a bulky methyl group it has more steric hindrance and is thus a worse nucleophile.
Explore Graduate Programs for You
Explore our featured graduate schools & programs to find those that both match your interests and are looking for students like you.
Best Law Schools
Check out our complete list of 168 law schools, based on surveys of school administrators and over 17,000 students.
Search for Medical Schools
Visit our Med School Hub to explore med schools with our ‘Find Your Med School’ filtered search or visit our Med School Advice pages for info about good MCAT scores or interview question prep.

Find MBA Programs Matched to Your Interests
Explore our featured business schools to find those that are looking for students like you.
Explore Graduate Programs for You
Explore our featured graduate schools & programs to find those that both match your interests and are looking for students like you.
Best Law Schools
Check out our complete list of 168 law schools, based on surveys of school administrators and over 17,000 students.
Search for Medical Schools
Visit our Med School Hub to explore med schools with our ‘Find Your Med School’ filtered search or visit our Med School Advice pages for info about good MCAT scores or interview question prep.

Find MBA Programs Matched to Your Interests
Explore our featured business schools to find those that are looking for students like you.
Explore Graduate Programs for You
Explore our featured graduate schools & programs to find those that both match your interests and are looking for students like you.
Best Law Schools
Check out our complete list of 168 law schools, based on surveys of school administrators and over 17,000 students.
Search for Medical Schools
Visit our Med School Hub to explore med schools with our ‘Find Your Med School’ filtered search or visit our Med School Advice pages for info about good MCAT scores or interview question prep.

Find MBA Programs Matched to Your Interests
Explore our featured business schools to find those that are looking for students like you.